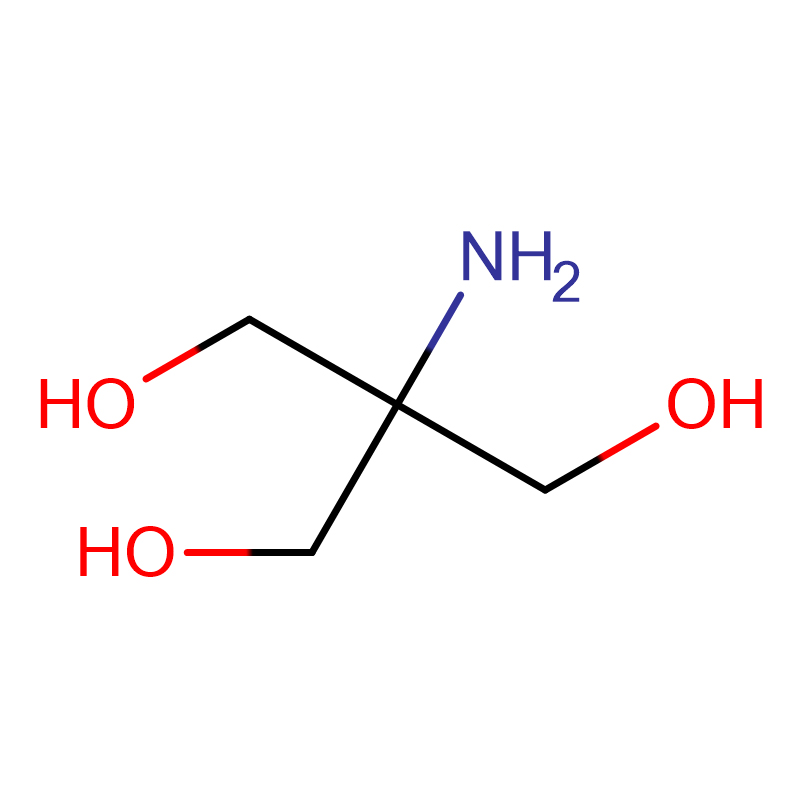
Tris Base Cas: 77-86-1 99.5% Farin ƙarfe mai ƙarfi
| Lambar Catalog | XD90056 |
| Sunan samfur | Tris Base |
| CAS | 77-86-1 |
| Tsarin kwayoyin halitta | Saukewa: C4H11NO3 |
| Nauyin Kwayoyin Halitta | 121.14 |
| Bayanin Ajiya | yanayi |
| Harmonized Tariff Code | 29221900 |
Ƙayyadaddun samfur
| Matsayin narkewa | 168.0°C - 172.0°C |
| Daraja | Babban darajar USP |
| Ruwa | <0.2% |
| Arsenic | 1 ppm max |
| Ganewa | IR ya dace |
| pH | 10.0 - 11.5 |
| Asara akan bushewa | 0.5% max |
| Solubility | A bayyane, mara launi |
| Assay | 99.5% min |
| Calcium | 3ppm ku |
| Iron | 5ppm ku |
| Copper | 1 ppm max |
| Ragowa akan Ignition | 0.1% max |
| Matsalolin da ba a iya narkewa | <0.03% |
| Karfe masu nauyi (Pb) | 5ppm ku |
| Chloride | 3ppm ku |
| Bayyanar | Farin kristal mai ƙarfi |
| Launi (20% maganin aq) | <5 |
| Identity Ph. Eur | Ya dace |
| Don amfani da bincike kawai, ba don amfanin ɗan adam ba | amfani da bincike kawai, ba don amfanin ɗan adam ba |
Bayani:Sunan alamar Tris shine tris (hydroxymethyl)aminomethane;tromethamine;tromethamine;2-amino-2- (hydroxymethyl) -1,3-propanediol.Farin crystal ne ko foda.Mai narkewa a cikin ethanol da ruwa, dan kadan mai narkewa a cikin ethyl acetate da benzene, wanda ba a iya narkewa a cikin ether da carbon tetrachloride, mai lalacewa zuwa jan karfe da aluminum, da sinadarai masu ban haushi.
Alamomi:Tromethamine tushe ne na amino buffer maras sodium, wanda ke amsawa da H2CO3 a cikin ruwan jiki don rage H2CO3 da samar da HCO32- a lokaci guda.Yana iya ɗaukar ions hydrogen kuma ya gyara acidemia.Ƙarfi, kuma yana iya shiga cikin tantanin halitta, wanda aka saba amfani dashi a cikin m metabolism da kuma numfashi acidemia.
Kaddarorin buffering:Tris tushe ne mai rauni tare da pKa na 8.1 a 25 ° C;bisa ga ka'idar buffer, ingantaccen kewayon buffer na Tris yana tsakanin pH 7.0 da 9.2.PH na maganin ruwa na tushen Tris shine kusan 10.5.Gabaɗaya, ana ƙara acid hydrochloric don daidaita ƙimar pH zuwa ƙimar da ake so, sannan ana iya samun maganin buffer tare da ƙimar pH.Duk da haka, ya kamata a biya hankali ga tasirin zafin jiki akan pKa na Tris.
Aikace-aikace:Ana amfani da Tris sosai a cikin m metabolism da kuma numfashi acidemia.Yana da buffer alkaline kuma yana da tasiri mai kyau na buffer akan metabolism acidosis da aikin enzymatic.Ana amfani da Tris sau da yawa azaman ma'ajin ilimin halitta kuma galibi ana tsara shi tare da ƙimar pH na 6.8, 7.4, 8.0, da 8.8.Tsarin tsarin sa da ƙimar pH sun bambanta sosai tare da zafin jiki.Gabaɗaya Littafin sinadarai ya ce ga kowane digiri na karuwa a zafin jiki, pH yana raguwa da 0.03.Ana amfani da Tris sosai a cikin shirye-shiryen buffers a cikin gwaje-gwajen nazarin halittu da kwayoyin halitta.Misali, ana buƙatar Tris a cikin duka TAE da TBE buffers (don solubilization na nucleic acid) waɗanda aka saba amfani da su a cikin gwaje-gwajen sinadarai.Tunda yana dauke da rukunin amino, yana iya jurewa halayen natsuwa tare da aldehydes.